Established GMP Quality Policy and Regulatory Support
Established GMP Quality Policy and Regulatory Support
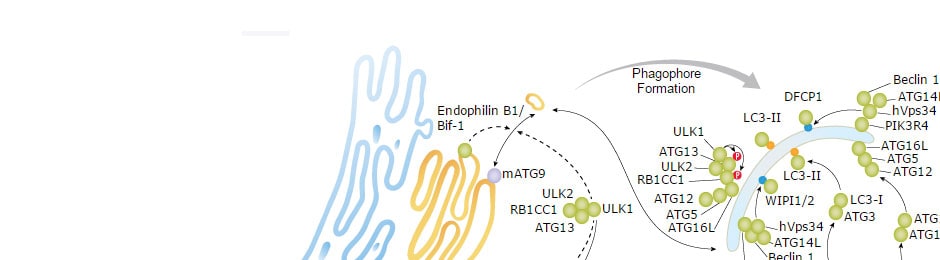
Experienced manufacturing of raw materials
Our teams have decades of experience in reagent development and IVD-certified manufacture, as well as a proven track record producing GMP-grade ancillary reagents for cell therapy clients.
Flexibility to fit into your processes
Start from an existing antibody or generate one from scratch. Customize vial size and formulation to streamline and de-risk your process. Scale up and scale out at your own pace. Tell us your needs – and we’ll customize your project.
Expert support for regulatory affairs
Use our dedicated team for support with process validation and regulatory affairs, to help your reagent transition from initial submission to market authorization. We’ll provide complete documentation every step of that way.
More from Bio-Techne
More from Bio-Techne